Thermoelectric
properties of boron-rich solids
Description of a specific
project for thermoelectric energy conversion
Thermoelectric Properties of Boron-Rich Solids and their Possibilities of Technical Application
(paper presented at the
25th International Conference on Thermoelectrics
August 6 - 10, 2006
Vienna, Austria)
H. Werheit
D-47048
E-mail
address:
helmut.werheit@koeln.de
Abstract
The thermoelectric properties of beta-rhombohedral boron and boron carbide, the best-investigated icosahedral boron-rich solids, are reviewed. Because of its high density of gap states (~1021 cm-3) generated by intrinsic defects, p-type boron carbide behaves electronically extrinsic up to at least 2000 K, and therefore it exhibits excellent thermoelectric performance. This can even be considerably improved by suitable interstitial doping (Si, Al). As the possibility of n-doping of boron carbide can be largely excluded, other n-type counterparts are required for technical application. Some alkaline hexaborides (Takeda et al.) and rare-earth boron carbonitrides (Mori et al.) are shown to be promising candidates to close this gap.
Introduction
Boron-rich solids are characterized by a large variety of complex structures essentially consisting of nearly regular polyhedra like B12 icosahedra, B12 dodecahedra, B6 octahedra and related structural elements. These are bonded directly to one another or via single boron or non-boron atoms thus forming open frameworks allowing the insertion of foreign atoms by substitution for regular B atoms or by interstitial accommodation. Moreover, intrinsic vacancies and structural defects play an essential role for the electronic properties (see [[1],[2],[3]] and references therein). Till now, only few of these materials have been sufficiently investigated to discuss their thermoelectric properties.
The suitability of materials for thermoelectric devices is to be checked on the basis of the theoretical efficiency
(1)
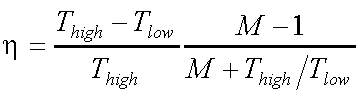
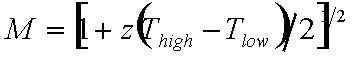
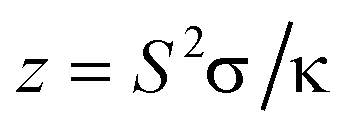
To optimize the factor (Thigh - Tlow)/ Thigh which is the theoretical efficiency of a Carnot machine, requires materials allowing application at very high temperatures. In this respect, boron-rich solids are outstanding materials because of their high melting points Tm typically exceeding 2000 K, e.g. boron carbide with Tm ≈ 2600 K, and metal hexaborides with Tm > 2500 K.
The factor (M-1)/M+Thigh/Tlow) essentially depends on z containing the transport properties, which are relevant for thermoelectrical application. In particular must be obeyed that towards high temperatures semiconductors usually become intrinsic. This means that their charge transport is essentially determined by electrons and holes, both thermally excited across the band gap. In this temperature range doping becomes ineffective, the Seebeck effects of electrons and hole largely compensate each other and hence the resulting efficiency becomes rather small. The resulting sign of S depends on the type of the more mobile carriers.
beta-rhombohedral boron.
This tendency towards intrinsic behavior determines the high-temperature thermoelectric properties of beta-rhombohedral boron, one of the few boron-rich semiconductors investigated in some detail. Fig. 1 shows that suitable n-doping of this originally p-type semiconductor can lead to considerable negative Seebeck coefficients; however, unfortunately at lower temperatures only. As shown in Fig. 2, at higher temperatures the electronic transport approaches intrinsic behavior with a comparably low positive S [[4],[5]].

Fig.
1
.
beta-rhombohedral
boron. Seebeck coefficient of pure and doped
beta-rhombohedral
boron at room temperature (Slack et al. [
4
], for Werheit et al., Kuhlmann et al., Schmechel et al., and further
examples of doped
b-rhombohedral
boron, see [1
] and references therein).
Doping of beta-rhombohedral boron with metal atoms leading to n-type conduction is limited because the number of suitable interstitial sites and there possible occupancies are restricted. For this reason, there seems to be no chance to accommodate n-doping elements in sufficient concentration to pin the Fermi level near the conduction band at high temperatures. Therefore, beta-rhombohedral boron must be largely excluded as a candidate for high-efficiency thermoelectric application.
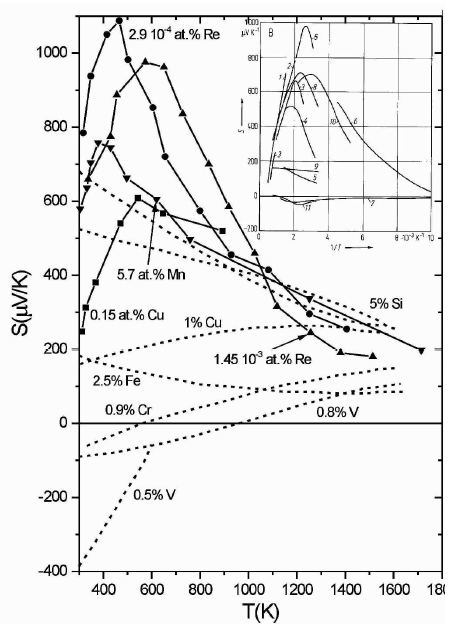
Fig.
2
.
beta-rhombohedral
boron. Seebeck coefficient of doped
beta-rhombohedral
boron vs. temperature; broken lines [4
], symbols [5
]. Insert, Seebeck coefficient of pure and
doped boron vs. 1/T (for details and references, see [[6]]
Boron carbide exhibits a large homogeneity
range extending from B4.3C at the carbon-rich to about B11C
at the boron-rich limit. There is no well-defined unit cell representing
the whole structure but only rhombohedral elementary cells composed of
twelve-atomic icosahedra at the vertex, and mostly three-atomic chains
on the main diagonal, both in different statistically distributed
compositions. There are B12 and B11C icosahedra,
and linear CBC, CBB, B□B
and sometimes CCC arrangements, whose concentrations depend on the
actual chemical composition (see [2],[[7],[8]]).
Compared with the idealized, according to theoretical calculations
energetically most favorable hypothetic structure B13C2
(structure formula B12(CBC) [[9],[10]],
metallic character), the real semiconducting structures of boron carbide
contain high concentrations of vacancies and antisite defects. These are
quantitatively correlated with the high concentration of gap states (~1021cm-3),
split off from the valence band and responsible for the semiconducting
character of real boron carbide [[11],[12]],
and in particular for the outstanding thermoelectric properties as well.
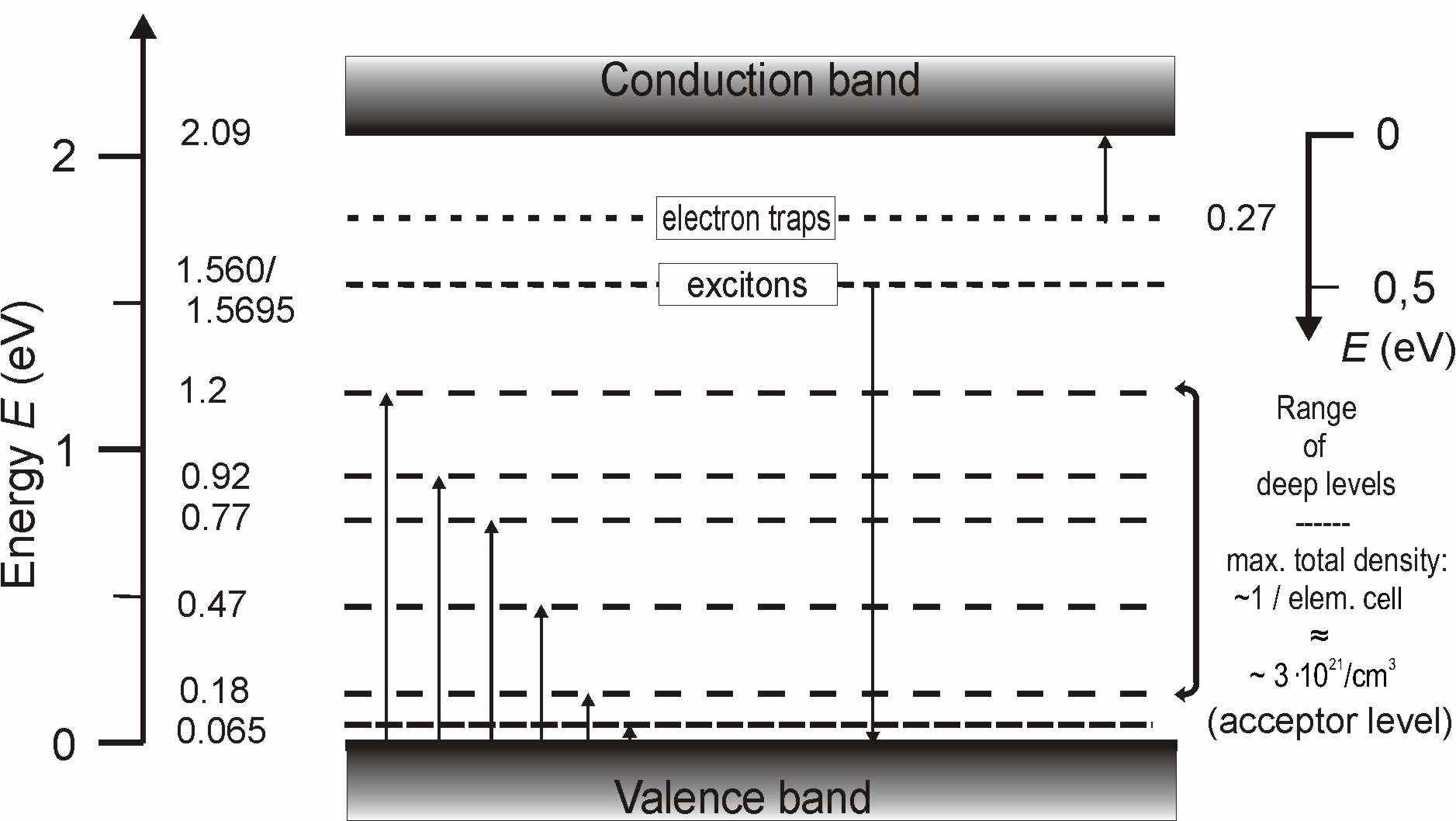
Fig.
3
.
Boron carbide. Preliminary band scheme
based on optical absorption, luminescence,
photoabsorption, and transport properties. The maximum total density of
defect-generated gap states is of the order 1 per unit cell, i.e. 3 1021
cm-3 [2
,11
,12
,
13 ,14
,
16 ]. The experimentally determined transitions are indicated by arrows.
An additionally predicted donor level [
11 ,
12
], which has not yet been experimentally
verified, is not marked.
A
preliminary band scheme of boron carbide is displayed in Fig. 3. It is
based on results of optical absorption, luminescence, photoabsorption,
electrical conductivity [2
,[13],[14],[15],[16]].
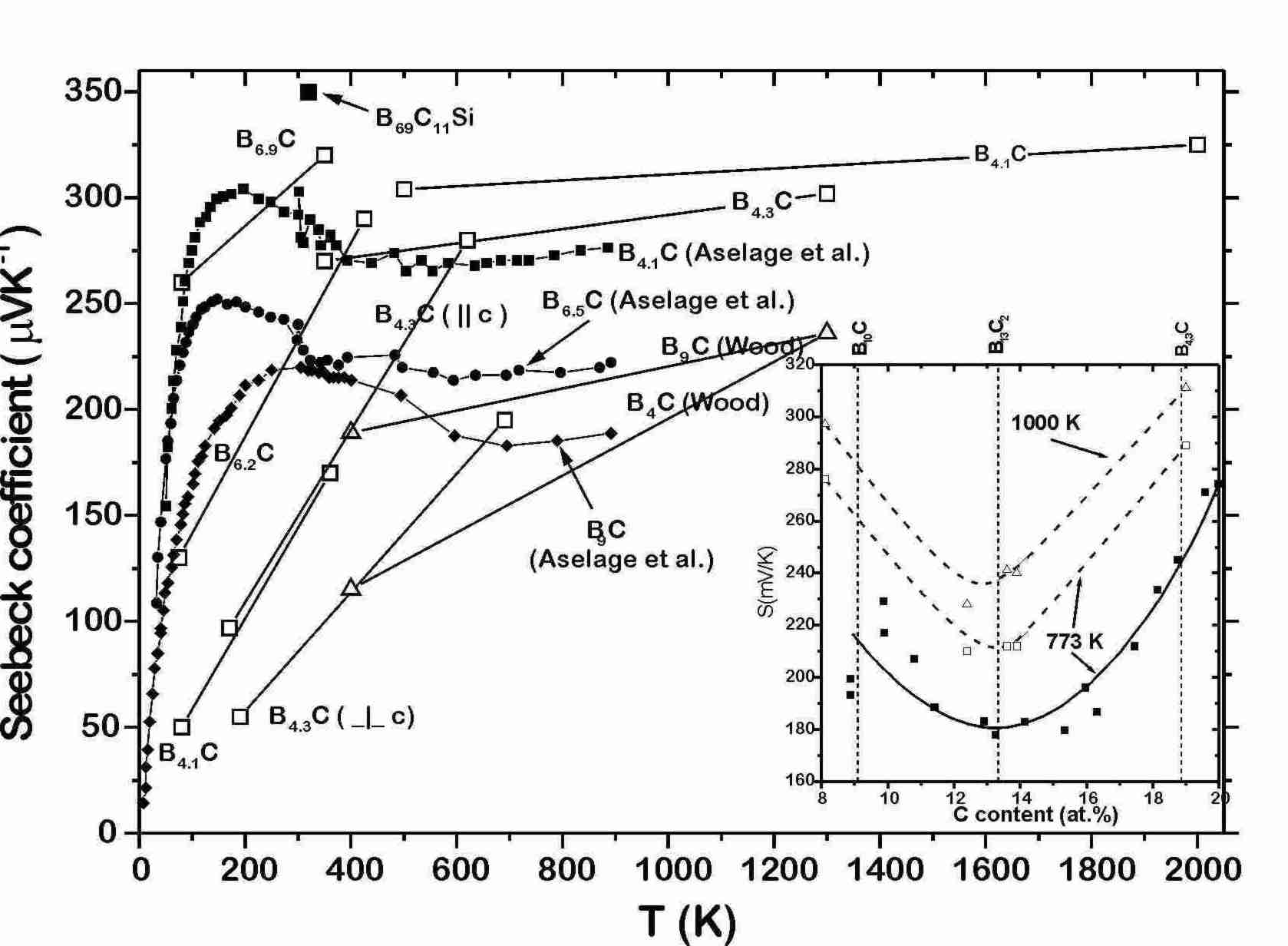
Some selected results of temperature and composition dependent Seebeck effect measurements on boron carbide are displayed in Fig. 4 [2,[18],[19],[20].[21]]. The essential result is that S weakly varies within the homogeneity range; minimum close to B13C2, and monotonously increases up to at least 2000 K. There is no remarkable indication of intrinsic behavior in this range. Reason is the extraordinary high concentration of gap states generated by structural defects [11] and pinning the Fermi level up to very high temperatures.

Fig.
5
.
Boron carbide. Electrical conductivity
plotted vs. T-1/4 according to Mott’s theory of
variable-range hopping [
2 ,
3
]. Insert,
plot vs. T-1 showing thermally activated processes at high
temperatures [[22]].
Fig. 5 shows the electrical
conductivity of boron carbide plotted according to Mott’s law of
variable range hopping, which law is obviously satisfied in a large
range of
temperature. This transport obviously takes
place within the high-density gap states. At high temperatures,
thermally activated carriers remarkably contribute to the charge
transport (see insert of Fig. 5). This superposition of hopping and band
conduction was separately proved at lower temperatures by analyzing the
dynamical transport of boron carbide determining the FIR spectra [[23],[24]].
In the whole homogeneity range the thermal conductivity (Fig. 6) is very low, and it remains low up to the limit of the hitherto available measurements at about 1100 K [[25],[26],[27],3 ]. Its magnitude and behavior is similar to that of glasses. This is not surprising, when the enormous density of structural defects is taken into account.
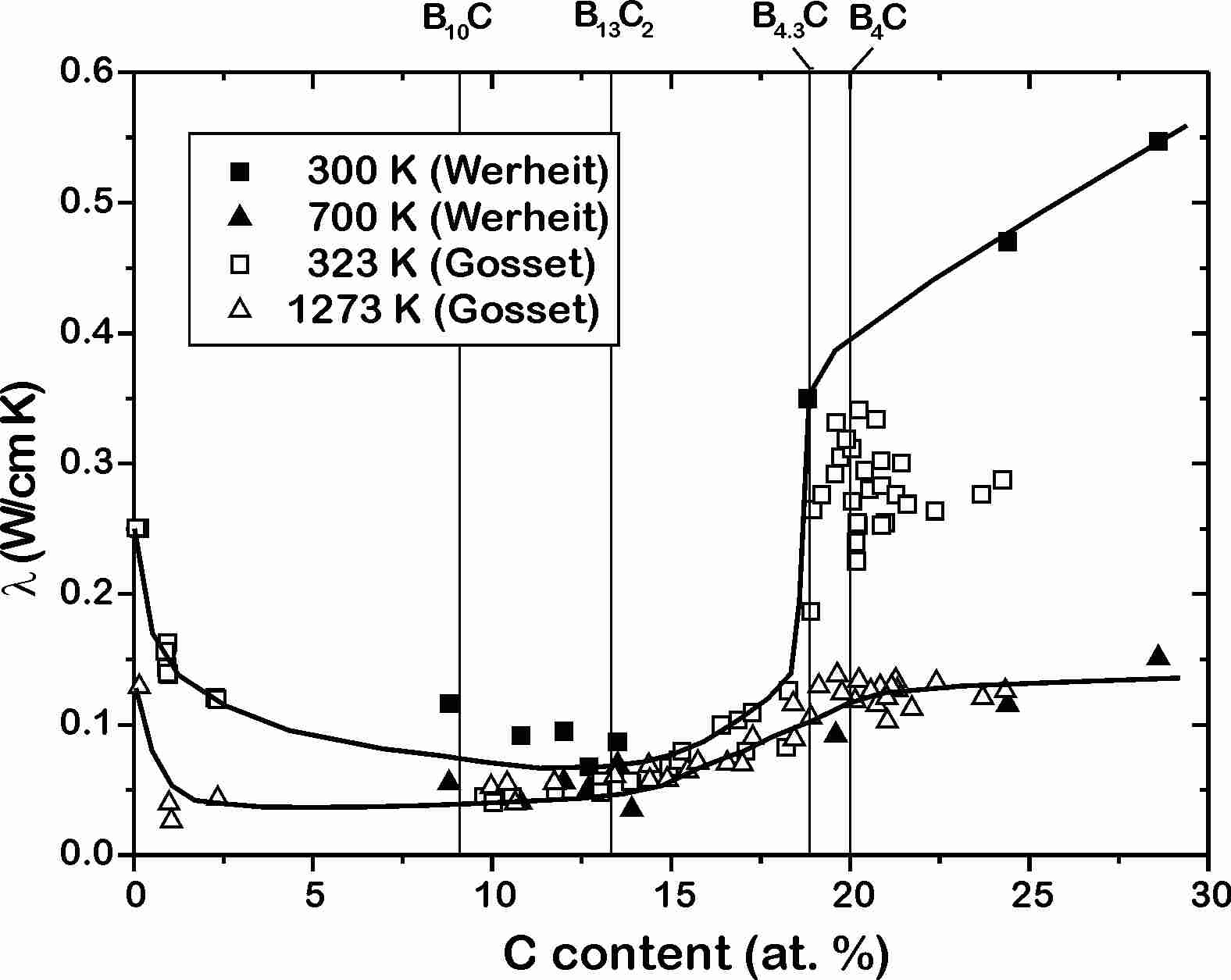
Fig.
6
Boron
carbide, carbon-doped boron. Thermal conductivity vs. carbon content [25
,26
,3].
Insert, Temperature dependence of the thermal conductivity of boron
carbide [27
].
The total effect of the thermoelectric properties is represented by the figure of merit z or by the dimensionless value zT (Fig. 7) essentially determining the efficiency η in equation (1). Obviously boron carbide is a very promising candidate for thermoelectric energy conversion.
Fig.
7
.
Boron
carbide. Figure of merit
z
and
zT
vs. temperature. Data below 1200 K were
calculated using the average experimental thermal conductivity; for
higher T it is linearly extrapolated.
However, for thermoelectric
devices with the high efficiency according to equation (1) a
corresponding n-type counterpart is required. In classical
semiconductors this problem is usually solved by suitable doping.
For boron carbide attempts of doping were
reported for numerous elements (for H, He, Mg, C, Si, N, P, O, Al, Cr,
Fe, see [2
] and references therein; for Fe, V, P
see [[28]]).
Some of the doping elements hitherto investigated increase
S
considerably. Si [[29],[30]]
(for
S
of nominal B69C11Si,
see Fig. 4) and Al
[[31]]
are accommodated in the chain-free (B□B)
elementary cells of boron carbide, only, and therefore their maximum
concentration is limited. For Al, the concentration-dependent
S
is shown in Fig. 8.
Till now,
n-type boron carbide has not been realized. Taking the high
concentration of gap states of up to about 3·1021 cm-3
(see Fig. 3) into account, this is not surprising, because a
corresponding overcompensation is required by substitutional or
interstitial accommodation of foreign atoms. Such a high concentration
of foreign atoms would probably destroy the boron carbide structure.
However, Hwang et al. [[32]]
report on n-type nickel-doped boron carbide thin films, but their result
has not been confirmed by other authors.
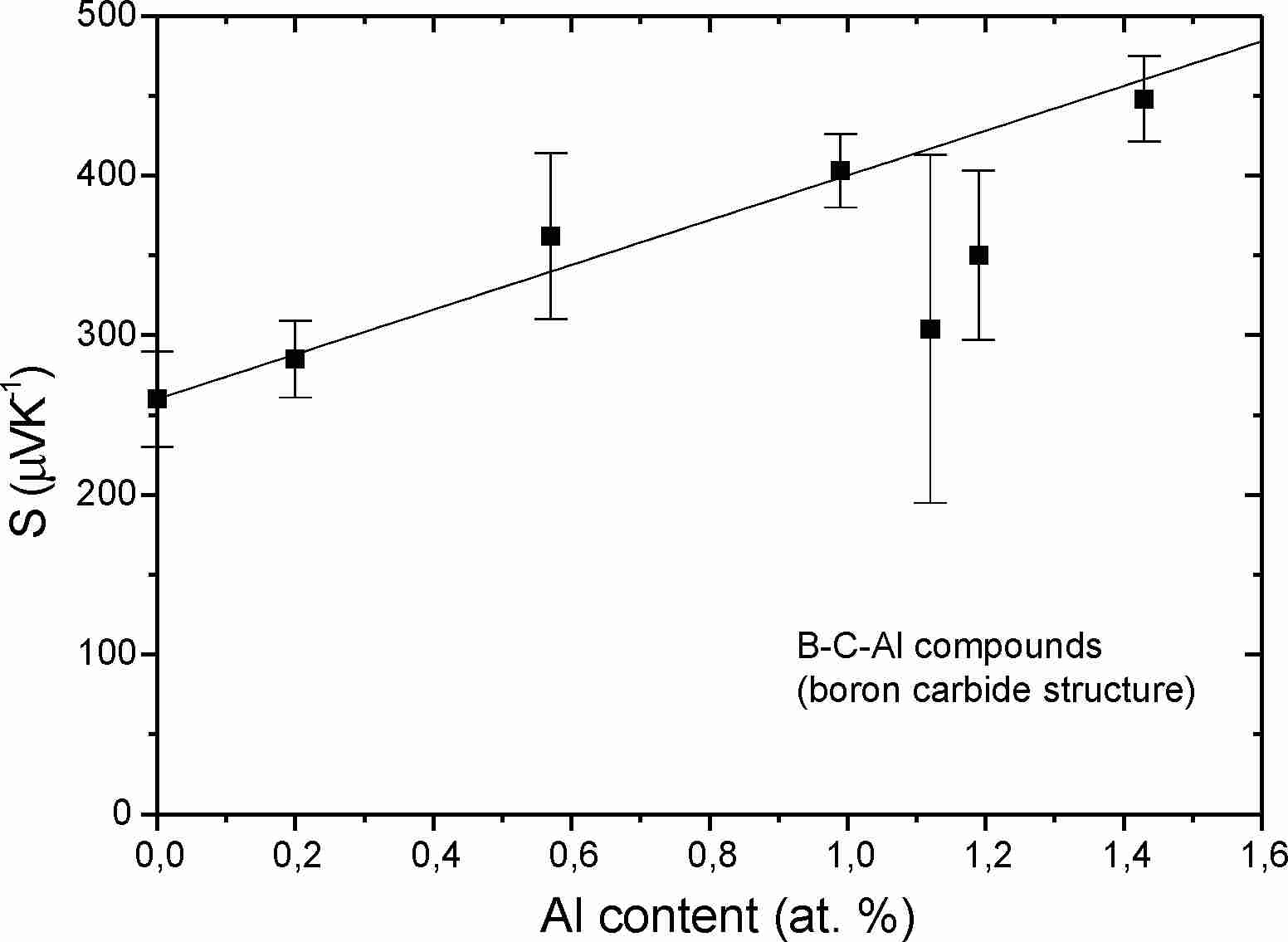
Fig.
8
.
Seebeck coefficient of Al-doped boron carbide
[31
]
n-type boron-rich solids
For beta-rhombohedral boron, the p-type behaviour is due to split-off valence states in the gap generated in consequence of structural defects [11 ,12 ] like in boron carbide. It was shown that, at least in particular cases, where the concentration of gap states is sufficiently low, p-type icosahedral boron-rich solids can be transferred to n-type by suitable interstitial doping (see above). In beta-rhombohedral boron, interstitial doping is limited by the restricted number of possible interstitial sites for the accommodation of metal atoms. As the electronic band structure of beta-rhombohedral boron is essentially determined by the B12 icosahedra of the structure, it seems to be useful to try other structure families containing B12 icosahedra as determining structural elements as well, but at least partly allowing much higher metal contents. In this connection are to be mentioned the
-
tetragonal boron structure groups,
-
orthorhombic γ-AlB12 structure group,
-
REB50 type borides
-
AlB10/C4AlB24 structure group
-
MgAlB14 type borides
-
YB25 structure group
-
YB66 type borides
Unfortunately, till now for all these structure groups measurements of thermoelectric properties have been performed in single cases at most, but never in systematic investigations (see [2 ]). Negative Seebeck coefficients were reported for MgAlB14, Al8B4C7, YbB6 and ErAlB14 (see [2 ] and references therein).
Indeed, recently some very promising thermoelectric investigations on some other n-type boron-rich solids have been performed:
Takeda et al. [[33],[34],[35]] have proved that alkaline hexaborides (CaB6, SrB6, and BaB6) exhibit considerable negative Seebeck coefficients monotonously increasing up to at least 1100 K, and with other promising thermoelectric properties up to very high temperatures as well.
Mori and Nishimura [[36],[37]] investigated HoB17CN, REB22C2N (RE=Y, Er, Lu), and YB28.5C4 containing B6 octahedra, B12 icosahedra and C-B-C chains as structural elements. Seebeck coefficients of the rare earth boron carbonitrides have a negative sign, and increase monotonously up to the limit of previous measurements at about 1100 K and other rather favorable thermoelectric properties.
Conclusions
Because of their outstanding general properties like high melting
points, extreme hardness and strong resistance against chemical attack,
boron-rich solids are excellent materials for application under extreme
external conditions, which are not accessible for many other materials.
In particular, this holds in the case of devices for thermoelectric
energy conversion, which require a high efficiency for their economic
use, as well. As shown, for the realization of this goal, besides of
favourable thermoelectric properties, the utilisation of highest
possible temperatures is a necessary prerequisite.
Though n-type doping of boron carbide has proved to be impossible, the construction of devices for high-efficiency thermoelectric energy conversion can be realized, when the p-type boron carbide is combined with n-type alkaline hexaborides or n-type RE boron carbonitrides respectively.
References
[1].
Werheit, H., Schmechel, R.,
Boron, in: O. Madelung (ed.),
Landolt-Börnstein, Numerical Data and Functional Relationships
in Science and Technology,
Group III, Vol. 41C, Springer,
[2]. Werheit, H., Boron compounds, in: O. Madelung (ed.), Landolt-Börnstein, Numerical Data and Functional Relationships in Science and Technology, New Series, Group III, Vol. 41D, Springer, Berlin, 2000, pp. 1 – 491.
[3].
Werheit, H., Boron and
Boron-rich Compounds, in Y. Kumashiro (ed.),
Electric refractory materials,
Marcel Dekker,
[4]. Slack, G.A., Rosolowski, J.H., Hejna, C., Garbauskas, M., Kasper, J.S., Proc. 9th Int. Symp.Boron, Boride and Rel. Comp., Univ. Duisburg, Germany, 1987, (H. Werheit, ed.), pp. 132-141.
[5]. Golikova O.A., Amandzhanov N., Kazanin, M.M., Klimashin, G.M., Kutasov, V.V., Phys. Stat. Sol. (a) 121 (1990) 579.,
[6].
Werheit, H., Boron, in: O.
Madelung (ed.), Landolt-Börnstein, Numerical Data and Functional
Relationships in Science and Technology, Group III, Vol.
17e,
Springer,
[7]. Werheit, H., Rotter, H.W., Meyer, F.D., Hillebrecht, H., Shalamberidze, S.O., Abzianidze, T.G., Esadze, G.G., FT-Raman spectra of isotope-enriched boron carbide, J. Solid State Chem. 177 (2004) 569-574.
[8].
Werheit, H., Leithe-Jasper,
A., Tanaka, T., Rotter, H.W., Schwetz, K.A., Some properties of
single-crystal boron carbide,
J. Solid State Chem. 177
(2004) 575-579.
[9]. Bylander, D.M., Kleinman, L., Lee, S., Self-consistant calculations of the energy bands and bonding properties of B12C3 , Phys. Rev. B 42 (1990) 1394.
[10]. Bylander, D.M., Kleinman, L., Structure of B13C2, Phys. Rev. B 43 (1991) 1487.
[11]. Schmechel, R., Werheit, H., Correlation between structural defects and electronic properties of icosahedral boron-rich solids, J. Phys.: Condens. Matter 11 (1999) 6803-6813.
[12].
Schmechel, R., Werheit, H.,
J. Solid State Chem. 154
(2000) 61-67.
[13]. Werheit, H., Binnenbruck, H., Hausen, A., Optical properties of boron carbide and comparison with β-rhombohedral boron, phys. Stat. sol. (b) 47 (1971) 153-158.
[14].
Schmechel, R., Thesis,
[15].
Schmechel, R., Werheit, H.,
Kampen, T.U., Mönch, W., Photoluminescence of boron carbide,
J. Solid State Chem.177 (2004)
566-568.
[16] Werheit, H., On excitons and other gap states in boron carbide, submitted to J. Phys: Condensed Matter.
[17].
Werheit, H., Kuhlmann, Franz,
R., Winkelbauer, W. Herstell, B., Fister, D., Neisius, H.,
Electronic transport properties of boron carbide within the
homogeneity range, in Emin, D., Aselage, T.L., Switendick, A.C.,
Morosin, B., Beckel, C.L. (eds.),
AIP Conf. Proc. 231,
Albuquerque, NM 1990, p. 104-107.
[18].
Wood, C.; Transport properties
of boron carbide, in Emin,D.; Aselage, T., Beckel, C.L., Howard,
I.A., Wood, C.,
AIP Conf. Proc. 140,
Albuquerque, New Mexico, 1985, pp. 206-215.
[19].
Wood, C.; Boron carbides as
high temperature thermoelectric materials, in Emin,D.; Aselage,
T., Beckel, C.L., Howard, I.A., Wood, C.,
AIP Conf. Proc. 140,
Albuquerque, New Mexico, 1985, pp. 362-372..
[20].
Aselage, T., Emin, D.,
McCready, S.S.,
[21].
Aselage, T., Emin, D.,
McCready, S.D.,
Phys. Rev. B 64 (2001) 54302.
[22]. Wood, C., The transport properties of boron carbide prepared by different techniques, in Proc. 9th Int. Symp.Boron, Boride and Rel. Comp., Univ. Duisburg, Germany, 1987, (H. Werheit, ed.), pp. 213-222.
[23].
Schmechel, R., Werheit, H.,
Evicence of the superposition of Drude type and hopping type
transport in boron-rich solids,
J. Solid State Chem. 133
(1997) 335-341.
[24].
Schmechel, R., Werheit, H.,
Dynamical transport in icosahedral boron-rich solids,
J. Mat. Proc. and manufact. Sci.
6 (1998) 329-337.
[25].
Gosset, D., Guery, M., Kryger,
B., Termal properties of some boron-rich compounds (“BnC”
and AlB12) in Emin, D., Aselage, T.L., Switendick,
A.C., Morosin, B., Beckel, C.L. (eds.),
AIP Conf. Proc. 231,
Albuquerque, NM 1990, pp. 380-383.
[26].
Kuhlmann, U., Werheit, H.,
Properties of boron carbide related to the carbon distribution
in the u nit cell, in Uno, R., Higashi, I. (eds.),
Proc. 11th Int. Conf.
Boron, Borides and Rel. Comp. (JJAP series 10), Tsukuba, Japan,
1993, pp.84-85.
[27].
Werheit, H., Kuss, N.,
unpublished results.
[28].
Aoki, Y., Miyazaki, Y., Okino,
Okinaka, N., Yatu, S., Kayukawa,, N., Takahashi, H., Hirai, T.,
Omori, M., Abe, Y., Study of B4C-Fe, B4C-V
and B4C-P Thermoelectric materials made by SPS for
MHD power system,
Proc. 33rd Plasmadynamics
and Laser Conf. 20-23 May 2002,
Maui, Hawaii., AIAA-2002-2213.
[29].
Werheit, H., Kuhlmann, U.,
Laux, M., Telle, R., Structural and optical properties of
Si-doped boron carbide, in Uno, R., Higashi, I. (eds.),
Proc. 11th Int. Conf.
Boron, Borides and Rel. Comp. (JJAP series 10), Tsukuba, Japan,
1993, pp. 86-87.
[30].
Werheit, H., Kuhlmann, U., Laux, M., Telle, R., Solid Solutions
of Silicon in Boron-Carbide-Type Crystals, J. Alloys Comp. 209,
181 (1994).
[31]
Schmechel, R., Werheit, H., Robberding, K., J. Solid State Chem.
133 (1997) 254-259.
[32].
Hwang, S.-D., Yang, K.,
Dowben, P.A., Ahmad, A.A., Ianno, N.J., Li, J.Z., Lin, J.Y.,
Jiang, H.X., McIlroy, D.N., Fabrication of n-type nickel doped
B5C1-δ homojunction and heterojunction diodes,
Appl. Phys. Lett. 70 (1997)
1028-1030.
[33]. Takeda, M., Fukuda, T., Domingo, F., Miura, T., thermoelectric properties of some metal borides, J. Solid State Chem. 177 (2004) 471-475.
[34].
Takeda, M., Terui, M.;
Takahashi, N., Ueda, N., Improvement of thermoelectric
properties of alkaline-earth hexaborides,
J. Solid State Chem. (Proc. 15th.
Int. Sympos. Boron, Borides and rel. Materials,
[35] Takeda, M., Takahashi, N., Structural analysis and thermoelectric properties of alkaline-earth hexaborides, this volume.
[36].
Mori, T., Nishimura, T.,
Thermoelectric properties of homologous p- and n-type boron-rich
borides,
J. Solid State Chem. (Proc. 15th.
Int. Sympos. Boron, Borides and rel. Materials,
[37] Mori, T., Nishimura, T., Homologous rare earth boron cluster compounds; a possible n-type counterpart to boron carbide, this volume.