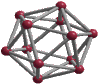
Structures of some boron-rich structures

Boron carbide
Symmetry type of
the structure is
![]() (space group 166). Initially, the rhombohedral unit cell was
assumed to be composed of B12 icosahedra at its vertices and
a linear CCC chain on the main diagonal parallel to the crystallographic
c-axis,
leading to the chemical composition B12C3.
After the central chain atom was proved to be solely boron, the
structure formula (B12) CCC was modified to (B11C)
CBC. This structure model is depicted in Fig. 1 using the program VESTA[i].
The apparently plausible formula B4C is long since proved to
be incorrect
7,
but nevertheless it has often been used as a synonym for boron carbide.
(space group 166). Initially, the rhombohedral unit cell was
assumed to be composed of B12 icosahedra at its vertices and
a linear CCC chain on the main diagonal parallel to the crystallographic
c-axis,
leading to the chemical composition B12C3.
After the central chain atom was proved to be solely boron, the
structure formula (B12) CCC was modified to (B11C)
CBC. This structure model is depicted in Fig. 1 using the program VESTA[i].
The apparently plausible formula B4C is long since proved to
be incorrect
7,
but nevertheless it has often been used as a synonym for boron carbide.
According to the present state of art, the
chemical compound B4C or B12C3
does not exist, at least not, when it is prepared by the common
high-temperature preparation methods melting or hot-pressing, typically
exceeding 2300 K. Actual chemical compounds of boron carbide exist in
the large homogeneity range extending from the carbon-rich limit B4.3C
[ii]
to the boron-rich limit B~10.8C
[iii].
Various structure elements have been identified; their concentrations
depend on the actual chemical composition, and the distribution is
assumed to be statistical, as no superlattice has been identified so
far:
·
B11C
icosahedra with the C atom accommodated in one of the 6 polar sites
·
CBC chains
·
CBB chains
·
B□B arrangements (□, vacancy)
Therefore, the most prominent methods of structure analysis, x-ray as
well as neutron scattering and NMR, fail in determining the actual
microstructure of boron carbide, indeed for different reasons. X-ray and
neutron diffraction, averaging a large volume with differently composed
elementary cells, yield the atomic sites correctly, but are unable to
determine their individual occupancies. Moreover, these methods are
decisively impeded by the difficulty of distinguishing B and C atoms
because of their very similar scattering cross section.
NMR spectra of boron carbide contain the
overlapping resonances of 11B and 13C isotopes
respectively, whose local environments vary in the volume analyzed. This
aggravates decisively the general problem of NMR, whose interpretation
is unambiguous at most in neat structures. Otherwise, it depends on the
more or less arbitrarily chosen structure models used for fitting the
spectra. In the case of boron carbide, the random mixture of differently
composed cells and structure elements makes a reliable analysis of the
spectra nearly impossible.
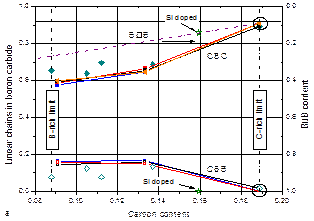

Concentration of structure elements of boron carbide vs. carbon content
a,
CBC, CBB and B□B (□, vacancy).
Isotopically pure BxC:
Full squares, CBC chains (derived from the stretching mode); open
squares, CBB chains (derived from the stretching mode); triangles,
CBC chains (derived from the Eu mode); dashed-dotted
line, average of chainless elementary cells (see Fig. 4).
natBxC:
Full diamonds, CBC; open diamonds,
CBB.
b.
B11C and B12 icosahedra.
Isotopically pure BxC:
Squares.
natBxC:
diamonds, this work;. Circles, results obtained from other natB4.3C
spectra
not shown here.
[i]
K. Momma and F. Izumi,
J. Appl. Crystallogr.
44,
1272 (2011).
[ii]
K. A. Schwetz and P. Karduck,
AIP Conf. Proc.,
231,
405 (1990).
[iii]
H. Werheit and S. Shalamberidze,
J. Phys.: Condens. Matter.,
24,
385406 (2012).